How Johnson and Johnson hip implants system went wrong
Health Ministry panel red-flags after-effects of Johnson & Johnson hip replacement systems, withdrawn worldwide. What are these complications, and how have India and other countries addressed them?

An expert committee set up by the Health Ministry has indicted Johnson & Johnson for “suppressing” key facts on the harmful effects of the company’s “faulty” hip replacement systems, withdrawn globally after complications required many patients to undergo revision surgery. A look at the concerns raised in India and elsewhere:
How is hip replacement done?
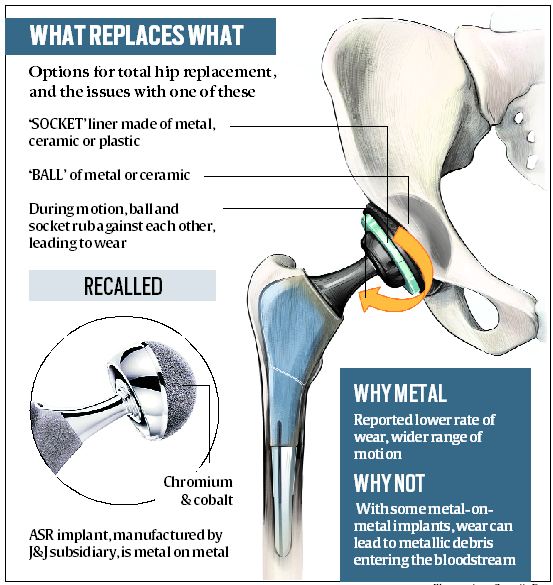
The hip joint consists of a ball and a socket, which are covered with cartilage and surrounded by a lubricating membrane to protect against wear. In total hip replacement, all components are replaced with prosthetic components. While a metal stem is placed into the hollow centre of the thighbone (femur), the prosthetic ball, socket and cartilage can be made of strong plastic, metal or ceramics. The commonest hip implants are metal on polythene, and ceramic on polythene.
What kind are the implants at the centre of the current controversy?
These are metal on metal, with cobalt, chromium and molybdenum as major constituents. Called ASR (Articular Surface Replacement) XL Acetabular System and ASR Hip Resurfacing System, these were being manufactured and sold for several years by Deputy International Limited (DePuy), UK, a subsidiary of Johnson & Johnson.
What problems arose with these?When the prosthetic ball and socket rub against each other, it causes wear. If the implant is metal on metal, this can sometimes releases metallic debris into the bloodstream. This can lead to complications, sometimes requiring revision surgery. Of the 93,000 patients implanted with ASR worldwide, many experienced serious adverse reactions, some requiring revision surgery to replace the ASR implant with another kind. Because of this, the company recalled the product on August 24, 2010.
To what extent has it happened in India?
In India, the company got the licence to import the device in 2006. By the time it was recalled worldwide, an estimated 4,700 ASR implants had been done in the country. Amid concerns worldwide, the Health Ministry set up an expert committee in 2017 to examine issues arising out of faulty ASR implants in India. Headed by Dr Arun Kumar Agarwal, former Dean and Professor of ENT at Maulana Azad Medical College, the committee reviewed action taken by the company to replace faulty ASR implants, and reviewed compensation provided to those who had suffered.
What did the committee find?
While more than 3,600 of the 4,700 patients could not be traced, the committee sent letters to 101, of whom 22 responded. The committee concluded that not only did patients undergo revision after first surgery, but “in some cases, more than one revision surgeries have been performed”.
About the adverse effects, it observed: “Some of the patients had reported that they had to undergo excoriating pain during all these and more particularly after the implant. Many patients reported general fatigue or local issues such pseudo tumour, pain walking, metallosis (increase in Cobalt and Chromium levels, Asthenozoospermia (reduced sperm motility), cyst in kidney, claudication pain.”
Besides physical problems, it noted: “Some of them informed that they are still having difficulty in carrying out their routine activities and are confined to bed which has led them to mental turmoil and agony. These patient also informed that the cost of revision surgery was reimbursed either by the company or the insurance firms. The patients are still sceptical about their future with the implant in their body.”
Has the committee suggested how these issues should be addressed?
The committee has recommended that:
* The company should be made liable to pay at least Rs 20 lakh to each patient with such complications, and the reimbursement programme be extended until August 2025.
* A central expert committee and a regional expert committee should be constituted by the Ministry for evaluation of patients’ claims in “respect of disability and suffering caused due to use of faulty ASR”. The regional committee will determine whether there is permanent disability, and whether such disability has affected or will affect the patient’s earning capacity, and then submit its report to the central expert committee.
* The central expert committee will determine the quantum of compensation. According to the committee that examined ASR implants, the base amount should be Rs 20 lakh, and in addition to this, the patient should be given compensation on the basis of suffering on “account of monetary loss due to wages and other loss” and percentage of disability. It has recommended that the maximum amount be at par with the maximum granted for clinical trial-related death and permanent disability as per rules and guidelines of the Drug Controller General of India.
* With 3,600 patients yet to traced, the “firm has to give due diligence to trace those remaining patients who have received ASR but have not registered with the helpline”.
* Health assessment of patients should be reported once a year till 2025 and compliance report periodically, preferably six-monthly, submitted to the Ministry. Follow-up should be done regularly.
* An independent registry should be established for tracking usage of high-risk medical devices. Provisions for compensation should be included in Medical Device Rules if any serious adverse event or death is caused due to the sole use of a medical device.
Was this the first time the government has examined the problem?
The first red flag had been raised in 2010, when the Maharashtra Food & Drug Administration (FDA) got an anonymous complaint about a patient suffering a serious adverse reaction, leading to an FDA investigation and an FIR with Mahim police. In 2012, the state FDA asked the regulator CDSCO to cancel the firm’s import licence for not taking proper remedial measures or creating awareness about the “defective”. CDSCO cancelled the product’s import and marketing in 2012, then issued a medical device alert on ASR implants in 2013 — three years after the global recall.
How have other countries addressed issues relating to ASR?
Australia, which had approved the product in 2004, was the first to take regulatory action against it. In 2007, an analysis by Therapeutic Goods Administration suggested that the ASR Hip Resurfacing System was associated with a higher-than-average replacement rate, as shown by Australian National Joint Replacement Registry data. In 2009, ASR was removed from the Australian market, By 2016, ASR had the highest revision rate for any hip implant used in Australia.
In the US, the National Institutes of Health in 2014 found that the ASR Hip Resurfacing System had the highest all-cause revision rate (24.2 % at 7 years) among resurfacing brands. It recommended continued clinical surveillance and laboratory monitoring of patients.
Source:-Indian Express
used here for education of general public only
No comments:
Post a Comment